News
Keep updated with the latest MDPP news via our newsletter.
The catheterisation of male patients is difficult and frustrating for urologists and, after years of contemplation, busy surgeon Ian Middleton decided he would help tackle the situation. Partnering with the Medical Device Partnering Program enabled Ian to develop a working prototype of his medical device and he is now seeking a catheter manufacturer for a pathway to production.
In his latest publication, Ian discusses the arduous process of patent acquisition - which is vital for innovation – and expresses his appreciation to the MDPP team for supporting and guiding him through the commercialisation pipeline.
Thanks for your kind words, Ian. We have enjoyed working with you!

Click here to read the paper: MiddletonPaper.PDF
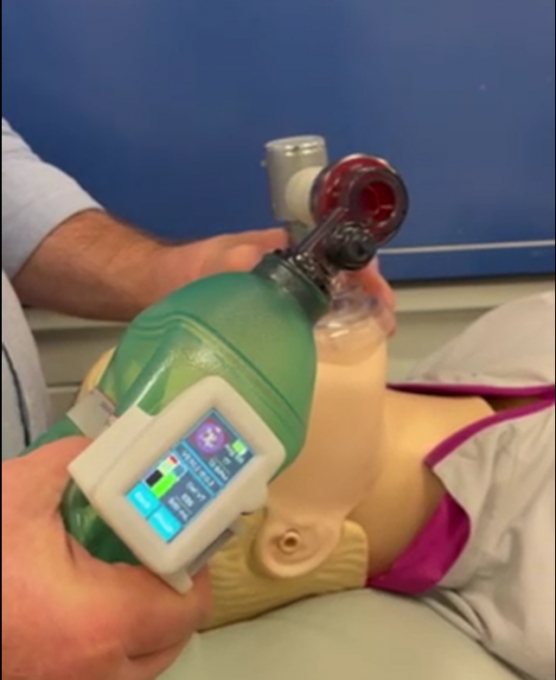
Ventilation management during life-saving resuscitation is being transformed by a local start-up, Abtulus®, who have partnered with the Medical Device Partnering Program (MDPP) at Flinders University to develop a novel Bag Valve Mask (BVM) guidance system.
Engineered by Dr Siavash Noor (Ahmadi Noorbakhsh) of Abtulus®, VentiWatch is a practical, simple and reusable ventilation management solution supporting medical professionals and first responders with the delivery of precise and efficient ventilation during resuscitation.
The cutting-edge sensor technology eliminates the common challenges associated with using BVMs, including inaccurate placement and incorrect technique of ventilation, which can cause further complications like delivering less than needed ventilations (hypoventilation) or pushing too much air into the lungs (hyperventilation) of patients in critical conditions.
The joint effort to advance CPR ventilation management was achieved with the MDPP team crafting a custom coil and sensing circuit, complete with sophisticated processing algorithm, to provide accurate estimates of air volume delivery and ventilation timing with real-time feedback.
Placed on the outside of the BVM, VentiWatch connects to the ventilation management device which features a user-friendly interface, colour LCD screen, navigation switch, audible alarms, a micro-USB port and a rechargeable lithium-ion battery.
First responders can then inflate a patient’s lungs with a squeeze of the BVM to obtain the perfect volume and timing for a successful resuscitation and VentiWatch’s continuous ventilation monitoring system then enables the first responders to safely transfer high-risk patients to hospital emergency departments.
 “The expertise, resources, and support provided by the MDPP team throughout the program have propelled my device and company to a whole new level,” remarked Dr Noor, Founder and CEO of Abtulus and inventor of VentiWatch.
“The expertise, resources, and support provided by the MDPP team throughout the program have propelled my device and company to a whole new level,” remarked Dr Noor, Founder and CEO of Abtulus and inventor of VentiWatch.
“Overall, working with MDPP has been an enriching experience. I look forward to a continued partnership as we bring this life-saving technology to the world.”
Professor Karen Reynolds, Director of MDPP, says VentiWatch has evolved the BVM system into a sophisticated tool with the potential to revolutionise CPR ventilation management.
“Through every stage of the project, our team of experts have consulted with Dr Noor, refining and optimising his initial design, while preserving its simplicity and reusability,” said Prof Reynolds.
“The BVM guidance system we have built for Dr Noor is the optimal choice in ventilation management support for first responders attending life-threatening emergencies.
We are extremely proud to be supporting the development of a novel medical device designed to deliver a solution that ensures optimal patient care during critical resuscitation scenarios.
The MDPP supports the development of new, high-tech medical devices through facilitating collaboration between researchers, industry, end- users and government, and undertaking rapid research projects that demonstrate proof of concept and de-risk ideas.

Sorry you missed this great event. Keep an eye on our website or Twitter for upcoming events.
You are invited to a seminar by guest presenter Prof Peter Hunter from University of Auckland
When: 10am on Monday 4th March
Where: Flinders University, 1284 South Road, Tonsley
Presentation title: A physics-based physiology approach for developing the virtual human twin
Professor Peter Hunter is a Distinguished Professor and founder of the Auckland Bioengineering Institute at the University of Auckland, co-Director of Computational Physiology at Oxford University and holds honorary or visiting Professorships at a number of Universities around the world. He is on the scientific advisory boards of a number of Research Institutes in Europe, the US and the Asia-Pacific region.
Professor Hunter's major research interests are on modelling many aspects of the human body using specially developed computational algorithms and an anatomically and biophysically based approach which incorporates detailed anatomical and microstructural measurements and material properties into the continuum models.
Please register via Eventbrite if you would like to attend.
The event will be livestreamed for those unable to attend in person.
Revolutionising the landscape of healthcare delivery, innovator Hayley Saddington has joined forces with Flinders University's Medical Device Partnering Program (MDPP) to build a cost-effective, clinically guided digital software application tailored for supporting in-home rehabilitation.
The co-founder of Peak Medical, Hayley is a visionary in her field who has exploited artificial intelligence to create an innovative technology which empowers in-home patients to progress their rehabilitation with the confidence of precise clinical guidance.
create an innovative technology which empowers in-home patients to progress their rehabilitation with the confidence of precise clinical guidance.
Replicating the physiotherapy experience, the technology employs an evidence-based clinical approach, directing individuals to customised exercises for continuous improvement between appointments.
Hayley says the idea for the transformative technology came to her as a practical solution in addressing the growing demand for physiotherapist support.
“We can’t scale up the number of human physiotherapists, but, with the help of MDPP we’ve been able to completely redefine the paradigm of care delivery,” said Hayley.
Allowing her to tap into “Australia’s critical startup ecosystem” Hayley says the MDPP provided valuable hands-on resources and connected her to university researchers, engineers, industry experts, and investors who helped bring her vision to life.
“The MDPP te am are exceptional, and they’ve got the recipe just right,” said Hayley. “With their expertise we navigated the shift from hardware to a software company, which was a smart play, enabling the rapid scalability and delivery of our low-cost healthcare app to a wider audience.”
am are exceptional, and they’ve got the recipe just right,” said Hayley. “With their expertise we navigated the shift from hardware to a software company, which was a smart play, enabling the rapid scalability and delivery of our low-cost healthcare app to a wider audience.”
According to Hayley, the MDPP’s 250 hours of engineering expertise has helped her to create a core technology with incredible expansion potential across various applications from tissue injury through to paediatrics.
“It is exciting for us to be a company that is transforming healthcare - not only in cost and accessibility - but actively empowering our patients to live their best lives,” said Hayley.
MDPP’s Director Professor Karen Reynolds is delighted to see Peak Medical now positioned as a significant player in the broader healthcare landscape and she is eager for other innovators who are keen to explore the synergies of technology and human-centric care to get in touch.
"By leveraging MDPP, innovators gain access to a robust startup ecosystem that validates their ideas and refines their strategies," said Karen. "Collaboratively, we can forge strategic partnerships that redefine the conventional patient experience and mould the future of global healthcare."
MaxM Skate's CEO, Rob Bowden, was interviewed by 5AA's Richard Pascoe on February 10 after attending Arab Health the Middle East’s largest healthcare trade event with 3,500 companies exhibiting from more than 180 countries around the world.
Health the Middle East’s largest healthcare trade event with 3,500 companies exhibiting from more than 180 countries around the world.
Have a listen to Rob and Richard as they discuss the benefits of using the MaxM Skate to achieve a faster in-home recovery after total knee replacement surgery.
Photos: Right - Rob Bowden with PhD Student Alicia Mitchell
Below - Rob Bowden and Alicia Mitchell with Prof Karen Reynolds (MDPP Director)


Sorry you missed this great event. Keep an eye on our website or Twitter for upcoming events.
The Medical Device Research Institute conducts world leading, collaborative research into Medical Devices to meet challenges facing the global healthcare sector.
You are invited to join us for the MDRI Showcase to hear from our key researchers and explore our labs and facilities at Flinders University at Tonsley on Wednesday 27 September 2023.
Program:
1:30pm – 2:30pm
Lecture Theatre 1, Ground Floor – Meet our key researchers and discover what we do.
- Rapid fire PhD presentations
- MDRI Industry collaboration success stories
- Learn about our mentoring program (and see how you can be involved)
2:30pm – 5pm
Explore our laboratories and facilities at your own pace and talk to our researches and students.
Visit our facilities:
- Medical Device Prototyping Lab
- Biomechanical/ Surgical Skills Lab
- Digital Health/CISCO Lab
- Rehab and Motion Analysis Lab
- Multimodal Recording Facility
- PPE Testing Facility
- MicroCT Lab

In the latest MedTech Mondays, we will be joined by experts with an understanding of how to scale up your medical device company.
Arjun Joshi - As Senior BDM at BSI, a notified body for CE Marking and various regulatory certifications for placing medical devices in various markets i.e. global market access, Arjun understands the requirements of ISO13485 and regulatory approvals process MDR, IVDR, MDSAP, UKCA, Article 117. Arjun also has a degree in Mechanical Engineering and an MBA and has held Operations Manager roles in companies previously. Additionally, Arjun has been working in the Medical devices industry for over 12 years where he has been a Lead Auditor for various standards such as Quality, OH&S, Environmental, SMETA (Ethical standard against modern slavery), etc.
Emma Bennett - Emma has over two decades working across a diverse cross section of industries, sectors and cultures, I have developed a vast collection of knowledge, skills and experience. Emma worked as General Manager of a company that manufactured IVF pipettes which was acquired by CooperSurgical. She also worked as Ops Manager of ISO13485 accredited electronics manufacturer Entech. Emma believes if we invest time in our employees and ourselves, through training and development, good work design and a continuous improvement culture, not only will the individual thrive but the organisation will prosper.
Chris Henry - Managing Director of Actis Medical, Chris runs an ISO 13485 certified company offering the medical device industry a distinct range of services. This includes medical device manufacturing, strategic sourcing to supplement med tech product portfolios and decentralised regulatory services embodying all regulatory affairs requirements.